
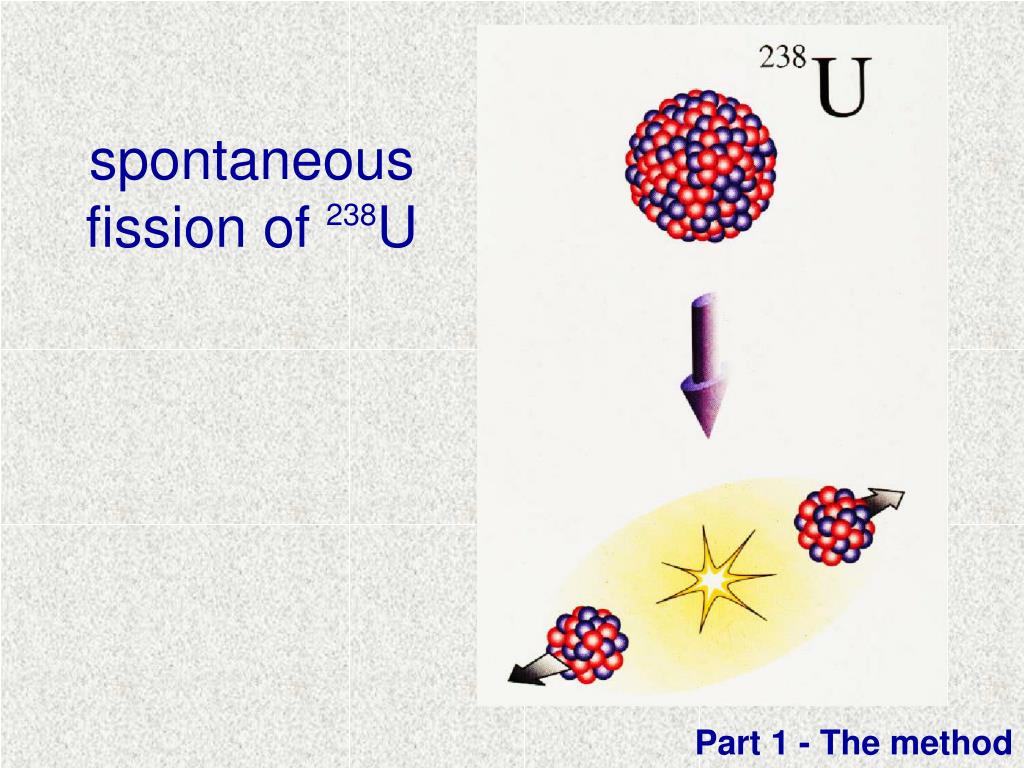
(Kalmthouts)įission is the opposite of fusion and releases energy only when heavy nuclei are split. The reactor is in the small domed building to the left of the towers. analysis of xenon gas concentration produced by spontaneous fission in the Primary Containment Vessel of Fukushima daiichi nuclear power plant unit1. The cooling towers are the most prominent features but are not unique to nuclear power. About 16 percent of the world's electrical power is generated by controlled nuclear fission in such plants. China is building nuclear power plants at the rate of one start every month.įigure 15.19 The people living near this nuclear power plant have no measurable exposure to radiation that is traceable to the plant. France provides over 75 percent of its electricity with nuclear power, while the United States has 104 operating reactors providing 20 percent of its electricity.
By the end of 2009, there were 442 reactors operating in 30 countries, providing 15 percent of the world's electricity. Whereas nuclear power was of little interest for decades following TMI and Chernobyl (and now Fukushima Daiichi), growing concerns over global warming has brought nuclear power back on the table as a viable energy alternative. Hundreds of nuclear fission power plants around the world attest to the fact that controlled fission is practical and, at least in the short term, economical, as seen in Figure 15.19. Controlled fission is a reality, whereas controlled fusion is a hope for the future. Nuclear fission is a reaction in which a nucleus is split (or fissured). 5.G.1.1 The student is able to apply conservation of nucleon number and conservation of electric charge to make predictions about nuclear reactions and decays such as fission, fusion, alpha decay, beta decay, or gamma decay.5.B.11.1 The student is able to apply conservation of mass and conservation of energy concepts to a natural phenomenon and use the equation E = m c 2 E = m c 2 to make a related calculation.4.C.4.1 The student is able to apply mathematical routines to describe the relationship between mass and energy and apply this concept across domains of scale.Fission Products can be produced by alpha, gamma, beta, charged particles, and through spontaneous reactions. 1.C.4.1 The student is able to articulate the reasons that the theory of conservation of mass was replaced by the theory of conservation of mass-energy. Below are scientific approaches to fission reactions, and the process which fission is produced.The information presented in this section supports the following AP® learning objectives and science practices: Describe controlled and uncontrolled chain reactions.Discuss how fission fuel reacts and describe what it produces.By the end of this section, you will be able to do the following:


 0 kommentar(er)
0 kommentar(er)
